Search
- Page Path
- HOME > Search
- Diabetes, obesity and metabolism
- Hypothalamic AMP-Activated Protein Kinase as a Whole-Body Energy Sensor and Regulator
- Se Hee Min, Do Kyeong Song, Chan Hee Lee, Eun Roh, Min-Seon Kim
- Endocrinol Metab. 2024;39(1):1-11. Published online February 14, 2024
- DOI: https://doi.org/10.3803/EnM.2024.1922
- 1,882 View
- 74 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
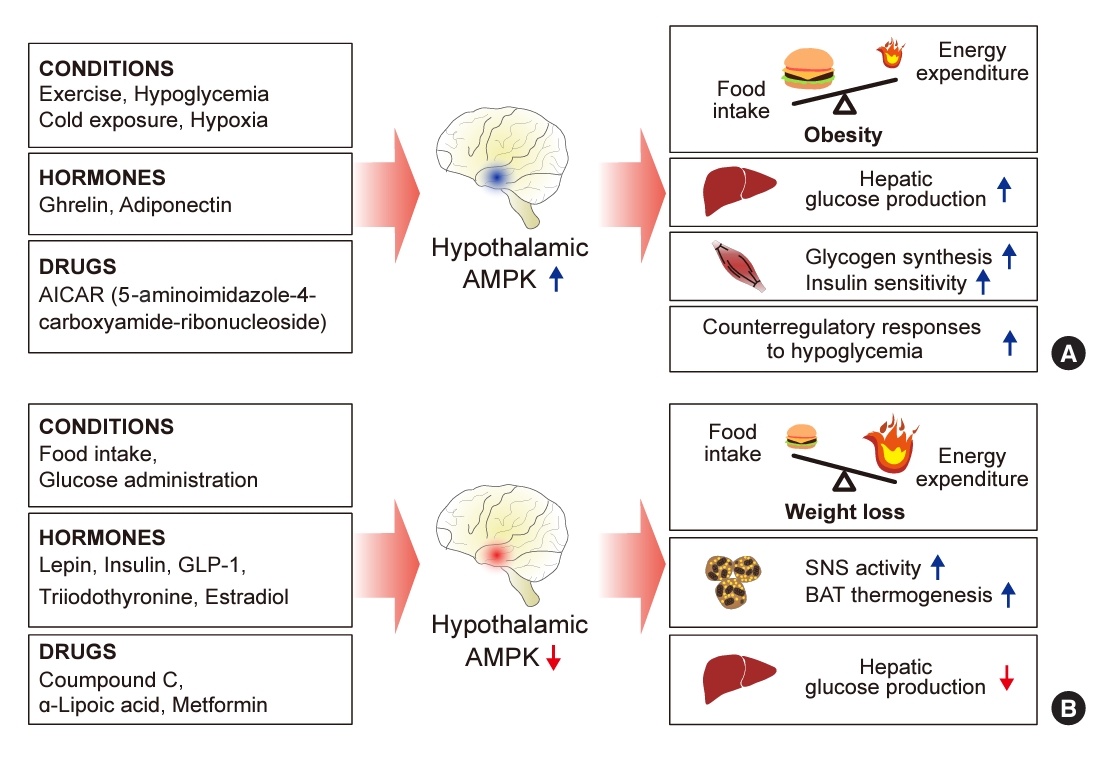
ePub - 5´-Adenosine monophosphate (AMP)-activated protein kinase (AMPK), a cellular energy sensor, is an essential enzyme that helps cells maintain stable energy levels during metabolic stress. The hypothalamus is pivotal in regulating energy balance within the body. Certain neurons in the hypothalamus are sensitive to fluctuations in food availability and energy stores, triggering adaptive responses to preserve systemic energy equilibrium. AMPK, expressed in these hypothalamic neurons, is instrumental in these regulatory processes. Hypothalamic AMPK activity is modulated by key metabolic hormones. Anorexigenic hormones, including leptin, insulin, and glucagon-like peptide 1, suppress hypothalamic AMPK activity, whereas the hunger hormone ghrelin activates it. These hormonal influences on hypothalamic AMPK activity are central to their roles in controlling food consumption and energy expenditure. Additionally, hypothalamic AMPK activity responds to variations in glucose concentrations. It becomes active during hypoglycemia but is deactivated when glucose is introduced directly into the hypothalamus. These shifts in AMPK activity within hypothalamic neurons are critical for maintaining glucose balance. Considering the vital function of hypothalamic AMPK in the regulation of overall energy and glucose balance, developing chemical agents that target the hypothalamus to modulate AMPK activity presents a promising therapeutic approach for metabolic conditions such as obesity and type 2 diabetes mellitus.

- Miscellaneous
- Immune Checkpoint Inhibitors and Endocrine Disorders: A Position Statement from the Korean Endocrine Society
- Hyemi Kwon, Eun Roh, Chang Ho Ahn, Hee Kyung Kim, Cheol Ryong Ku, Kyong Yeun Jung, Ju Hee Lee, Eun Heui Kim, Sunghwan Suh, Sangmo Hong, Jeonghoon Ha, Jun Sung Moon, Jin Hwa Kim, Mi-kyung Kim, The Committee of Clinical Practice Guideline of the Korean Endocrine Society
- Endocrinol Metab. 2022;37(6):839-850. Published online December 26, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1627
- 3,488 View
- 321 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
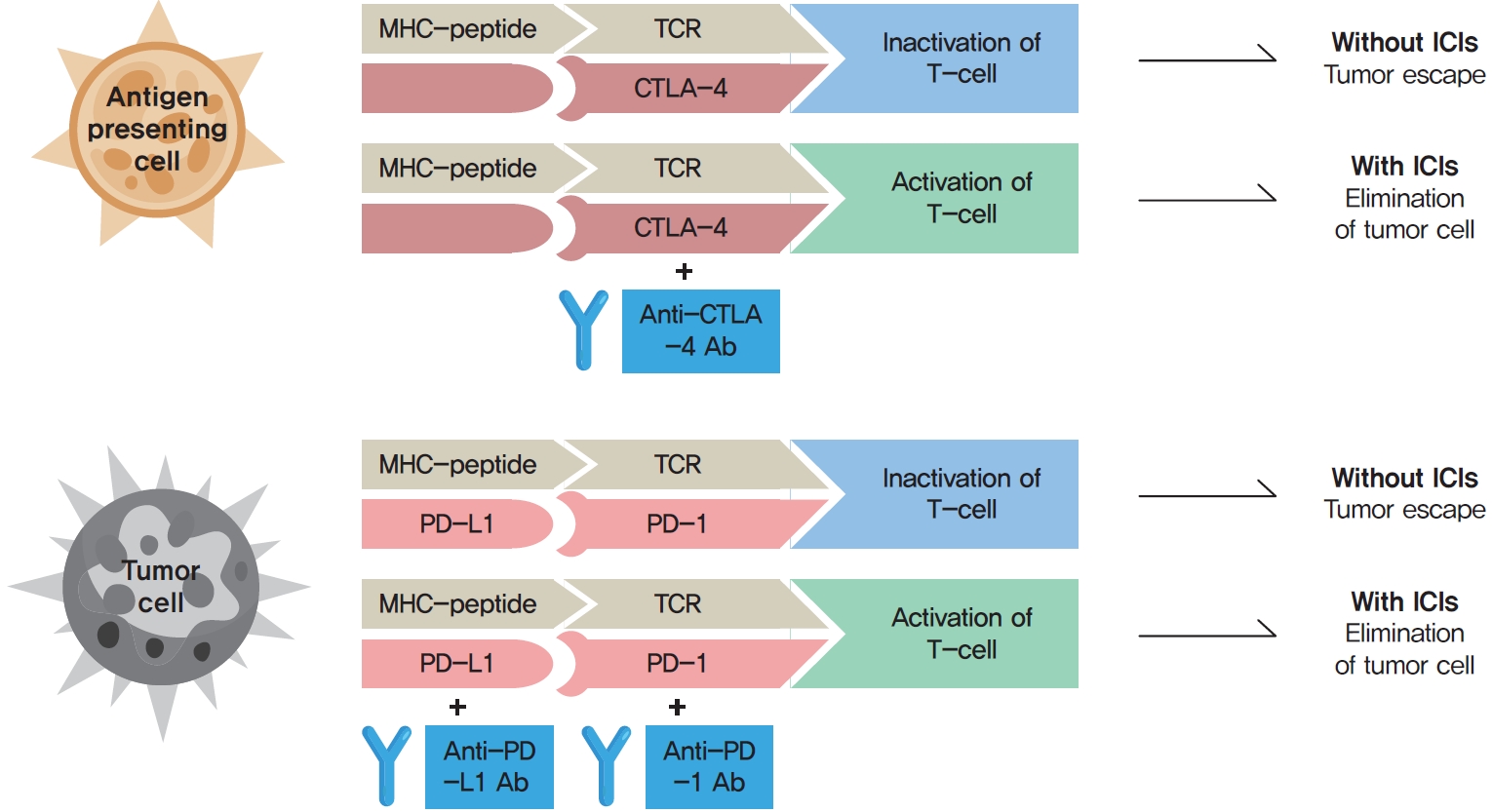
ePub - Immune checkpoint inhibitors (ICIs) including an anti-cytotoxic T-lymphocyte-associated antigen 4 inhibitor, anti-programmed cell death protein 1 (PD-1) inhibitors, and anti-PD-ligand 1 inhibitors are representative therapeutics for various malignancies. In oncology, the application of ICIs is currently expanding to a wider range of malignancies due to their remarkable clinical outcomes. ICIs target immune checkpoints which suppress the activity of T-cells that are specific for tumor antigens, thereby allowing tumor cells to escape the immune response. However, immune checkpoints also play a crucial role in preventing autoimmune reactions. Therefore, ICIs targeting immune checkpoints can trigger various immune-related adverse events (irAEs), especially in endocrine organs. Considering the endocrine organs that are frequently involved, irAEs associated endocrinopathies are frequently life-threatening and have unfavorable clinical implications for patients. However, there are very limited data from large clinical trials that would inform the development of clinical guidelines for patients with irAEs associated endocrinopathies. Considering the current clinical situation, in which the scope and scale of the application of ICIs are increasing, position statements from clinical specialists play an essential role in providing the appropriate recommendations based on both medical evidence and clinical experience. As endocrinologists, we would like to present precautions and recommendations for the management of immune-related endocrine disorders, especially those involving the adrenal, thyroid, and pituitary glands caused by ICIs.
-
Citations
Citations to this article as recorded by- Pembrolizumab plus lenvatinib for radically unresectable or metastatic renal cell carcinoma in the Japanese population
Ryo Fujiwara, Takeshi yuasa, kenichi kobayashi, tetsuya yoshida, susumu kageyama
Expert Review of Anticancer Therapy.2023; 23(5): 461. CrossRef - Incidence of Endocrine-Related Dysfunction in Patients Treated with New Immune Checkpoint Inhibitors: A Meta-Analysis and Comprehensive Review
Won Sang Yoo, Eu Jeong Ku, Eun Kyung Lee, Hwa Young Ahn
Endocrinology and Metabolism.2023; 38(6): 750. CrossRef
- Pembrolizumab plus lenvatinib for radically unresectable or metastatic renal cell carcinoma in the Japanese population

- Diabetes, Obesity and Metabolism
- Sestrin2 Regulates Beneficial β3-Adrenergic Receptor-Mediated Effects Observed in Inguinal White Adipose Tissue and Soleus Muscle
- Min Jeong Park, Joo Won Kim, Eun Roh, Kyung Mook Choi, Sei Hyun Baik, Hwan-Jin Hwang, Hye Jin Yoo
- Endocrinol Metab. 2022;37(3):552-557. Published online June 29, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1421
- 2,631 View
- 107 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
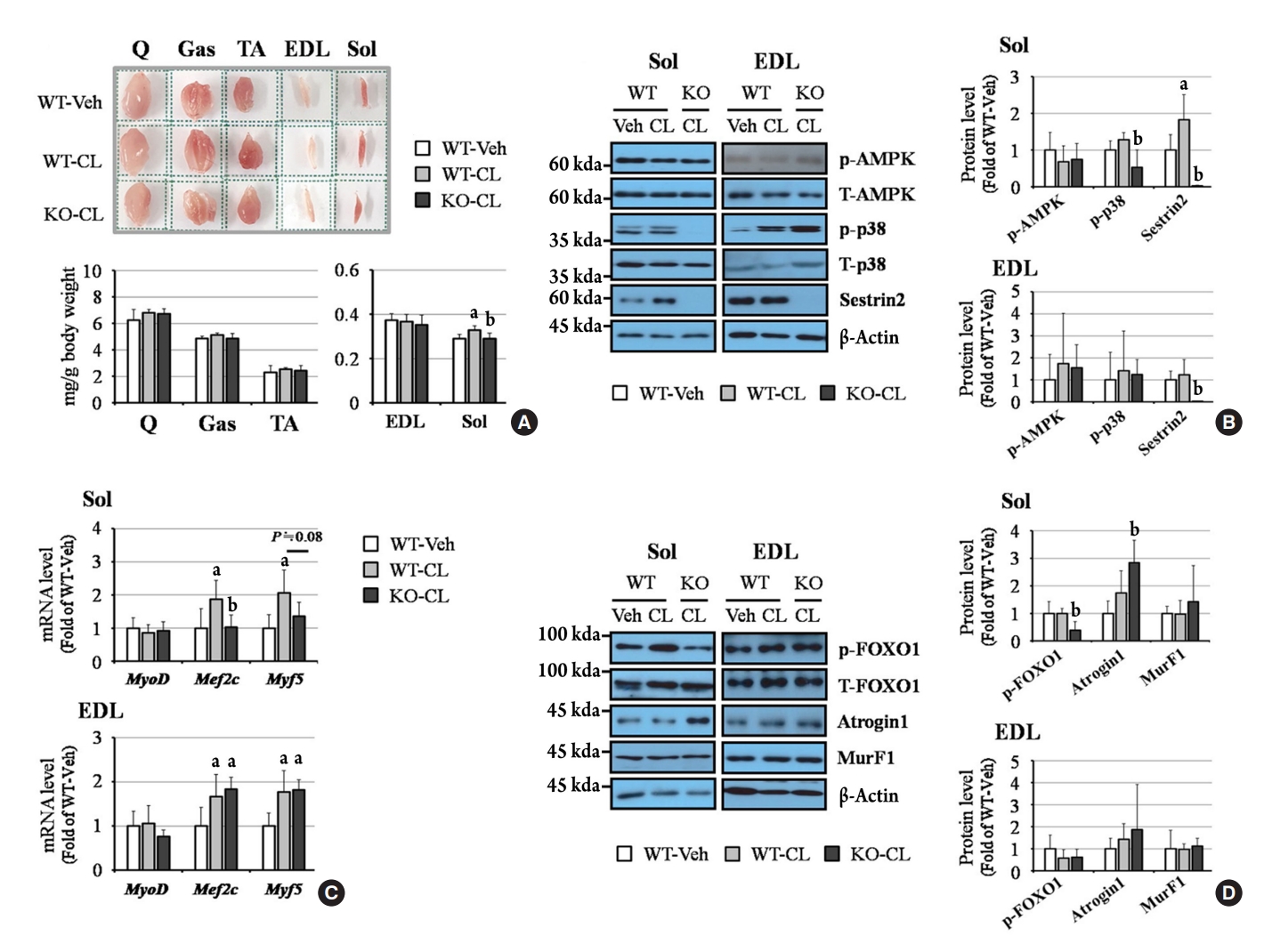
ePub - Sestrin2, a well-known adenosine monophosphate-activated protein kinase (AMPK) regulator, plays a protective role against metabolic stress. The β3-adrenergic receptor (β3AR) induces fat browning and inhibits muscle atrophy in an AMPK-dependent manner. However, no prior research has examined the relationship of sestrin2 with β3AR in body composition changes. In this study, CL 316,243 (CL), a β3AR agonist, was administered to wild-type and sestrin2-knockout (KO) mice for 2 weeks, and fat and muscle tissues were harvested. CL induced AMPK phosphorylation, expression of brown-fat markers, and mitochondrial biogenesis, which resulted in the reduction of lipid droplet size in inguinal white adipose tissue (iWAT). These effects were not observed in sestrin2-KO mice. In CL-treated soleus muscle, sestrin2-KO was related to decreased myogenic gene expression and increased levels of muscle atrophy-related molecules. Our results suggest that sestrin2 is associated with beneficial β3AR-mediated changes in body composition, especially in iWAT and in the soleus.
-
Citations
Citations to this article as recorded by- Sestrin2 levels in patients with anxiety and depression myocardial infarction was up-regulated and suppressed inflammation and ferroptosis by LKB1-mediated AMPK activation
Yufeng Qian, Lian Chen, Beibei Gao, Xianhua Ye
Clinical and Experimental Hypertension.2023;[Epub] CrossRef - Sestrin2 in diabetes and diabetic complications
Xiaodan Zhang, Zirui Luo, Jiahong Li, Yaxuan Lin, Yu Li, Wangen Li
Frontiers in Endocrinology.2023;[Epub] CrossRef
- Sestrin2 levels in patients with anxiety and depression myocardial infarction was up-regulated and suppressed inflammation and ferroptosis by LKB1-mediated AMPK activation

- Thyroid
Big Data Articles (National Health Insurance Service Database) - Risk of Diabetes in Patients with Long-Standing Graves’ Disease: A Longitudinal Study
- Eyun Song, Min Ji Koo, Eunjin Noh, Soon Young Hwang, Min Jeong Park, Jung A Kim, Eun Roh, Kyung Mook Choi, Sei Hyun Baik, Geum Joon Cho, Hye Jin Yoo
- Endocrinol Metab. 2021;36(6):1277-1286. Published online December 16, 2021
- DOI: https://doi.org/10.3803/EnM.2021.1251
- 5,176 View
- 181 Download
- 9 Web of Science
- 10 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
The detrimental effects of excessive thyroid hormone on glucose metabolism have been widely investigated. However, the risk of diabetes in patients with long-standing hyperthyroidism, especially according to treatment modality, remains uncertain, with few longitudinal studies.
Methods
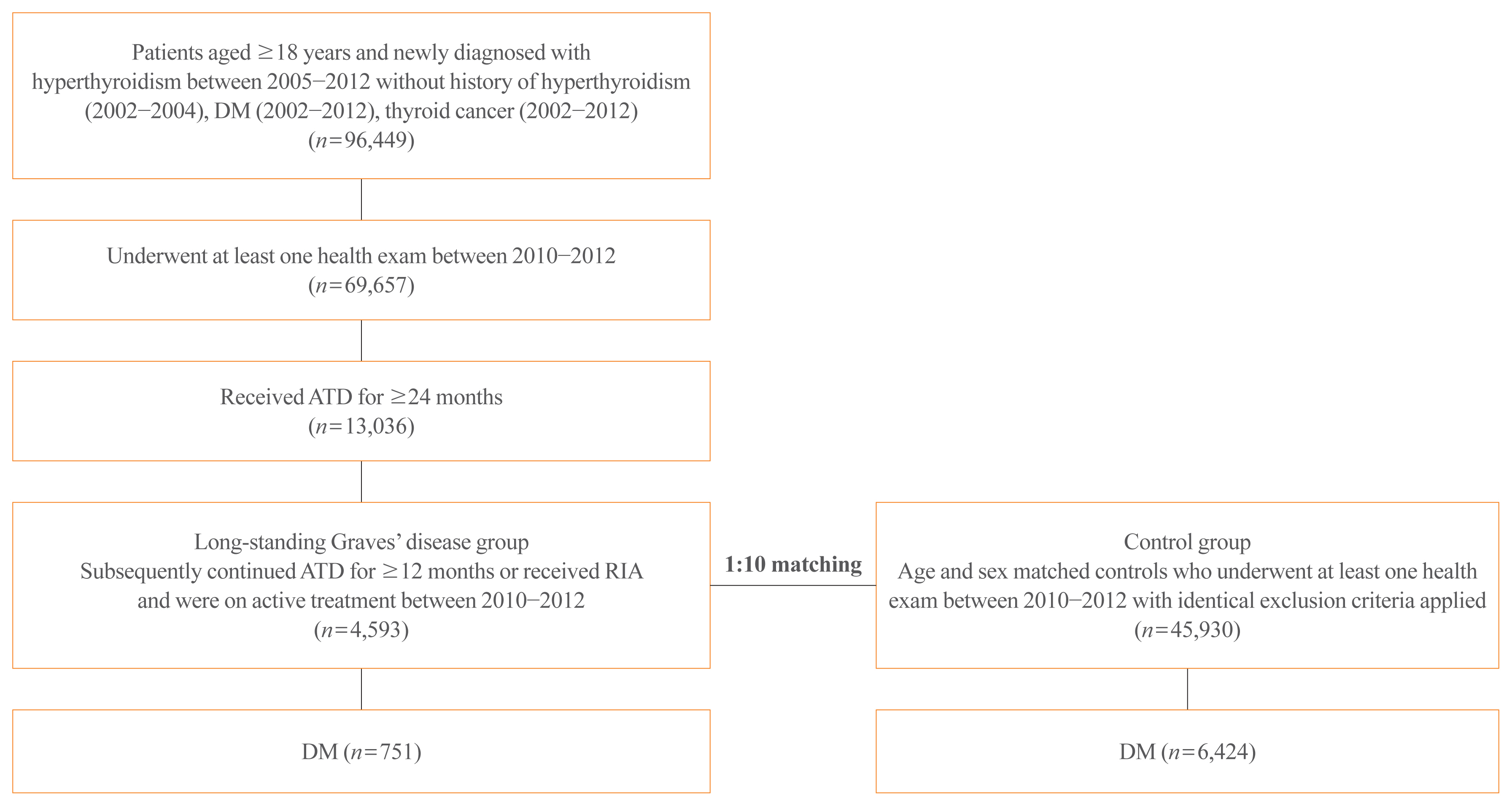
The risk of diabetes in patients with Graves’ disease treated with antithyroid drugs (ATDs) for longer than the conventional duration (≥2 years) was compared with that in age-and sex-matched controls. The risk was further compared according to subsequent treatment modalities after a 24-month course of ATD: continuation of ATD (ATD group) vs. radioactive iodine ablation (RIA) group.
Results
A total of 4,593 patients were included. Diabetes was diagnosed in 751 (16.3%) patients over a follow-up of 7.3 years. The hazard ratio (HR) for diabetes, after adjusting for various known risk factors, was 1.18 (95% confidence interval [CI], 1.10 to 1.28) in patients with hyperthyroidism. Among the treatment modality groups, the RIA group (n=102) had a higher risk of diabetes than the ATD group (n=4,491) with HR of 1.56 (95% CI, 1.01 to 2.42). Further, the risk of diabetes increased with an increase in the ATD treatment duration (P for trend=0.019).
Conclusion
The risk of diabetes was significantly higher in patients with long-standing Graves’ disease than in the general population, especially in patients who underwent RIA and prolonged ATD treatment. Special attention to hyperglycemia during follow-up along with effective control of hyperthyroidism may be necessary to reduce the risk of diabetes in these patients. -
Citations
Citations to this article as recorded by- Safety of non-standard regimen of systemic steroid therapy in patients with Graves’ orbitopathy: a single-centre experience
Nadia Sawicka-Gutaj, Dawid Gruszczyński, Natalia Zawalna, Kacper Nijakowski, Agnieszka Skiba, Mateusz Pochylski, Jerzy Sowiński, Marek Ruchała
Pharmacological Reports.2024; 76(1): 185. CrossRef - Increased risk of diabetes mellitus and hyperlipidemia in patients with differentiated thyroid cancer
Hwa Young Ahn, Jooyoung Lee, Jinmo Kang, Eun Kyung Lee
European Journal of Endocrinology.2024; 190(3): 248. CrossRef - Prevalencia de diabetes en personas con disfunción tiroidea
Juan J. Díez, Pedro Iglesias
Medicina Clínica.2023; 160(8): 333. CrossRef - Control of Thyroid Dysfunction in Spanish Population Registered in
the Primary Care Clinical Database: An Analysis of the Proportion of Patients
with Thyrotropin Values Outside the Reference Range
Juan J. Díez, Pedro Iglesias
Hormone and Metabolic Research.2023; 55(03): 184. CrossRef - Prevalence of thyroid dysfunction and its relationship to income level and employment status: a nationwide population-based study in Spain
Juan J. Díez, Pedro Iglesias
Hormones.2023; 22(2): 243. CrossRef - Prevalence of diabetes in people with thyroid dysfunction
Juan J. Díez, Pedro Iglesias
Medicina Clínica (English Edition).2023; 160(8): 333. CrossRef - Diabetes Mellitus Secondary to Endocrine Diseases: An Update of Diagnostic and Treatment Particularities
Mihaela Simona Popoviciu, Lorena Paduraru, Raluca Marinela Nutas, Alexandra Maria Ujoc, Galal Yahya, Kamel Metwally, Simona Cavalu
International Journal of Molecular Sciences.2023; 24(16): 12676. CrossRef - Thyroid Eye Disease and Its Association With Diabetes Mellitus: A Major Review
Roshmi Gupta, Pramila Kalra, Lakshmi B. Ramamurthy, Suryasnata Rath
Ophthalmic Plastic & Reconstructive Surgery.2023; 39(6S): S51. CrossRef - Metabolite Changes during the Transition from Hyperthyroidism to Euthyroidism in Patients with Graves’ Disease
Ho Yeop Lee, Byeong Chang Sim, Ha Thi Nga, Ji Sun Moon, Jingwen Tian, Nguyen Thi Linh, Sang Hyeon Ju, Dong Wook Choi, Daiki Setoyama, Hyon-Seung Yi
Endocrinology and Metabolism.2022; 37(6): 891. CrossRef - Diabetes and Hyperthyroidism: Is There a Causal Link?
Sang Yong Kim
Endocrinology and Metabolism.2021; 36(6): 1175. CrossRef
- Safety of non-standard regimen of systemic steroid therapy in patients with Graves’ orbitopathy: a single-centre experience

- Miscellaneous
- COVID-19 Vaccination for Endocrine Patients: A Position Statement from the Korean Endocrine Society
- Cheol Ryong Ku, Kyong Yeun Jung, Chang Ho Ahn, Jun Sung Moon, Ju Hee Lee, Eun Heui Kim, Hyemi Kwon, Hee Kyung Kim, Sunghwan Suh, Sangmo Hong, Jeonghoon Ha, Eun Roh, Jin Hwa Kim, Mi-kyung Kim, the Committee of Clinical Practice Guideline of the Korean Endocrine Society
- Endocrinol Metab. 2021;36(4):757-765. Published online August 17, 2021
- DOI: https://doi.org/10.3803/EnM.2021.404
- 10,360 View
- 419 Download
- 19 Web of Science
- 21 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Since the first outbreak of coronavirus disease 2019 (COVID-19), ongoing efforts have been made to discover an efficacious vaccine against COVID-19 to combat the pandemic. In most countries, both mRNA and DNA vaccines have been administered, and their side effects have also been reported. The clinical course of COVID-19 and the effects of vaccination against COVID-19 are both influenced by patients’ health status and involve a systemic physiological response. In view of the systemic function of endocrine hormones, endocrine disorders themselves and the therapeutics used to treat them can influence the outcomes of vaccination for COVID-19. However, there are very limited data to support the development of clinical guidelines for patients with specific medical backgrounds based on large clinical trials. In the current severe circumstances of the COVID-19 pandemic, position statements made by clinical specialists are essential to provide appropriate recommendations based on both medical evidence and clinical experiences. As endocrinologists, we would like to present the medical background of COVID-19 vaccination, as well as precautions to prevent the side effects of COVID-19 vaccination in patients with specific endocrine disorders, including adrenal insufficiency, diabetes mellitus, osteoporosis, autoimmune thyroid disease, hypogonadism, and pituitary disorders.
-
Citations
Citations to this article as recorded by- COVID-19 mRNA vaccine may trigger subacute thyroiditis
Mehmet Sözen, Ömercan Topaloğlu, Berrin Çetinarslan, Alev Selek, Zeynep Cantürk, Emre Gezer, Damla Köksalan, Taner Bayraktaroğlu
Human Vaccines & Immunotherapeutics.2024; 17(12): 5120. CrossRef - The role of co-morbidities in the development of an AEFI after COVID-19 vaccination in a large prospective cohort with patient-reported outcomes in the Netherlands
C. Ouaddouh, J.W. Duijster, T. Lieber, F.P.A.M. van Hunsel
Expert Opinion on Drug Safety.2024; 23(3): 323. CrossRef - Thyroid dysfunction in COVID-19
David Tak Wai Lui, Chi Ho Lee, Yu Cho Woo, Ivan Fan Ngai Hung, Karen Siu Ling Lam
Nature Reviews Endocrinology.2024;[Epub] CrossRef - Adult-Onset Type 1 Diabetes Development Following COVID-19 mRNA Vaccination
Hyeyeon Moon, Sunghwan Suh, Mi Kyoung Park
Journal of Korean Medical Science.2023;[Epub] CrossRef - Prior immunization status of COVID-19 patients and disease severity: A multicenter retrospective cohort study assessing the different types of immunity
Javaria Aslam, Faisal Shahzad Khan, Muhammad Talha Haris, Hewad Hewadmal, Maryam Khalid, Mohammad Y. Alshahrani, Qurrat-ul-ain Aslam, Irrum Aneela, Urooj Zafar
Vaccine.2023; 41(2): 598. CrossRef - Mortality and Severity of Coronavirus Disease 2019 in Patients with Long-Term Glucocorticoid Therapy: A Korean Nationwide Cohort Study
Eu Jeong Ku, Keeho Song, Kyoung Min Kim, Gi Hyeon Seo, Soon Jib Yoo
Endocrinology and Metabolism.2023; 38(2): 253. CrossRef - Pituitary Diseases and COVID-19 Outcomes in South Korea: A Nationwide Cohort Study
Jeonghoon Ha, Kyoung Min Kim, Dong-Jun Lim, Keeho Song, Gi Hyeon Seo
Journal of Clinical Medicine.2023; 12(14): 4799. CrossRef - Inactivated SARS-CoV-2 vaccination does not disturb the clinical course of Graves’ disease: An observational cohort study
Shichen Xu, Huixin Yu, Xian Cheng, Jing Wu, Jiandong Bao, Li Zhang
Vaccine.2023; 41(38): 5648. CrossRef - Adrenal Crisis Associated With COVID-19 Vaccination in Patients With Adrenal Insufficiency
Yukako Kurematsu, Takako Mohri, Sadanori Okada, Yutaka Takahashi
JCEM Case Reports.2023;[Epub] CrossRef - Adverse Events Associated with COVID-19 Vaccination in Adolescents with Endocrinological Disorders: A Cross-Sectional Study
İbrahim Mert Erbaş, İrem Ceren Erbaş, Gözde Akın Kağızmanlı, Kübra Yüksek Acinikli, Özge Besci, Korcan Demir, Ece Böber, Nurşen Belet, Ayhan Abacı
Journal of Clinical Research in Pediatric Endocrinology.2023; 15(3): 248. CrossRef - Neue Aspekte der Glukokortikoidsubstitution bei Nebennierenrindeninsuffizienz
Tina Kienitz, Gesine Meyer
Der Internist.2022; 63(1): 12. CrossRef - Endocrine Follow-up During Post-Acute COVID-19: Practical Recommendations Based on Available Clinical Evidence
Rimesh Pal, Ameya Joshi, Sanjay K. Bhadada, Mainak Banerjee, Suresh Vaikkakara, Satinath Mukhopadhyay
Endocrine Practice.2022; 28(4): 425. CrossRef - Safety of Inactivated and mRNA COVID-19 Vaccination Among Patients Treated for Hypothyroidism: A Population-Based Cohort Study
Xi Xiong, Carlos King Ho Wong, Ivan Chi Ho Au, Francisco Tsz Tsun Lai, Xue Li, Eric Yuk Fai Wan, Celine Sze Ling Chui, Esther Wai Yin Chan, Franco Wing Tak Cheng, Kristy Tsz Kwan Lau, Chi Ho Lee, Yu Cho Woo, David Tak Wai Lui, Ian Chi Kei Wong
Thyroid.2022; 32(5): 505. CrossRef - The New Entity of Subacute Thyroiditis amid the COVID-19 Pandemic: From Infection to Vaccine
Mihaela Popescu, Adina Ghemigian, Corina Maria Vasile, Andrei Costache, Mara Carsote, Alice Elena Ghenea
Diagnostics.2022; 12(4): 960. CrossRef - Adrenal Crisis Secondary to COVID-19 Vaccination in a Patient With Hypopituitarism
Nikolina Markovic, Anila Faizan, Chirag Boradia, Sridhar Nambi
AACE Clinical Case Reports.2022; 8(4): 171. CrossRef - The Effect of Inactivated SARS-CoV-2 Vaccines on TRAB in Graves’ Disease
LingHong Huang, ZhengRong Jiang, JingXiong Zhou, YuPing Chen, HuiBin Huang
Frontiers in Endocrinology.2022;[Epub] CrossRef - Osteoporosis in Patients With Respiratory Diseases
Yue Ma, Shui Qiu, Renyi Zhou
Frontiers in Physiology.2022;[Epub] CrossRef - Pilot Findings on SARS-CoV-2 Vaccine-Induced Pituitary Diseases: A Mini Review from Diagnosis to Pathophysiology
Ach Taieb, El Euch Mounira
Vaccines.2022; 10(12): 2004. CrossRef - Forty Years Together, New Leap Forward! The 40th Anniversary of the Korean Endocrine Society
Jong Chul Won, Ki-Hyun Baek
Endocrinology and Metabolism.2022; 37(6): 851. CrossRef - No need of glucocorticoid dose adjustment in patients with adrenal insufficiency before COVID-19 vaccine
Tania Pilli, Cristina Dalmiglio, Gilda Dalmazio, Alfonso Sagnella, Raffaella Forleo, Lucia Brilli, Fabio Maino, Cristina Ciuoli, Maria Grazia Castagna
European Journal of Endocrinology.2022; 187(1): K7. CrossRef - Diabetes and COVID-19 Vaccination
Hae Dong Choi, Jun Sung Moon
The Journal of Korean Diabetes.2021; 22(4): 221. CrossRef
- COVID-19 mRNA vaccine may trigger subacute thyroiditis

- Obesity and Metabolism
- Brain Regulation of Energy Metabolism
- Eun Roh, Min-Seon Kim
- Endocrinol Metab. 2016;31(4):519-524. Published online December 20, 2016
- DOI: https://doi.org/10.3803/EnM.2016.31.4.519
- 9,861 View
- 182 Download
- 53 Web of Science
- 53 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader In healthy individuals, energy intake is in balance with energy expenditure, which helps to maintain a normal body weight. The brain's inability to control energy homeostasis underlies the pathology of hyperphagia and obesity. The brain detects body energy excess and deficit by sensing the levels of circulating metabolic hormones and nutrients and by receiving metabolic information from the periphery via the autonomic nervous system. A specialized neuronal network coordinates energy intake behavior and the metabolic processes affecting energy expenditure. Here, we briefly review neuronal mechanisms by which our body maintains energy balance.
-
Citations
Citations to this article as recorded by- Hypothalamic AMP-Activated Protein Kinase as a Whole-Body Energy Sensor and Regulator
Se Hee Min, Do Kyeong Song, Chan Hee Lee, Eun Roh, Min-Seon Kim
Endocrinology and Metabolism.2024; 39(1): 1. CrossRef - Central inhibition of stearoyl-CoA desaturase has minimal effects on the peripheral metabolic symptoms of the 3xTg Alzheimer’s disease mouse model
Laura K. Hamilton, Paule E. H. M’Bra, Sophia Mailloux, Manon Galoppin, Anne Aumont, Karl J. L. Fernandes
Scientific Reports.2024;[Epub] CrossRef - Adipokines from white adipose tissue in regulation of whole body energy homeostasis
Bijayashree Sahu, Naresh C. Bal
Biochimie.2023; 204: 92. CrossRef - Growth hormone receptor (GHR) in AgRP neurons regulates thermogenesis in a sex-specific manner
Lukas Stilgenbauer, Juliana Bezerra Medeiros de Lima, Lucas Kniess Debarba, Manal Khan, Lisa Koshko, John J. Kopchick, Andrzej Bartke, Augusto Schneider, Marianna Sadagurski
GeroScience.2023; 45(3): 1745. CrossRef - Living high - training low model applied to C57BL/6J mice: Effects on physiological parameters related to aerobic fitness and acid-base balance
Pedro Paulo Menezes Scariot, Marcelo Papoti, Emanuel Elias Camolese Polisel, Juan Bordon Orsi, Paul R. Van Ginkel, Tomas A. Prolla, Fúlvia Barros Manchado-Gobatto, Claudio Alexandre Gobatto
Life Sciences.2023; 317: 121443. CrossRef - Whole Transcriptome Analysis of Hypothalamus in Mice during Short-Term Starvation
Eun-Young Oh, Byong Seo Park, Hye Rim Yang, Ho Gyun Lee, Thai Hien Tu, Sunggu Yang, Mi-Ryung Han, Jae Geun Kim
International Journal of Molecular Sciences.2023; 24(4): 3204. CrossRef - Hormonal Gut–Brain Signaling for the Treatment of Obesity
Eun Roh, Kyung Mook Choi
International Journal of Molecular Sciences.2023; 24(4): 3384. CrossRef - Neuronal Blockade of Thyroid Hormone Signaling Increases Sensitivity to Diet-Induced Obesity in Adult Male Mice
Eva Rial-Pensado, Laurence Canaple, Romain Guyot, Christoffer Clemmensen, Joëlle Wiersema, Shijia Wu, Sabine Richard, Anita Boelen, Timo D Müller, Miguel López, Frédéric Flamant, Karine Gauthier
Endocrinology.2023;[Epub] CrossRef - Genetic Contributors to Obesity
Ramya Sivasubramanian, Sonali Malhotra
Gastroenterology Clinics of North America.2023; 52(2): 323. CrossRef - Neurocomputational mechanisms of food and physical activity decision-making in male adolescents
Seung-Lark Lim, Amanda S. Bruce, Robin P. Shook
Scientific Reports.2023;[Epub] CrossRef - Induction of a torpor-like hypothermic and hypometabolic state in rodents by ultrasound
Yaoheng Yang, Jinyun Yuan, Rachael L. Field, Dezhuang Ye, Zhongtao Hu, Kevin Xu, Lu Xu, Yan Gong, Yimei Yue, Alexxai V. Kravitz, Michael R. Bruchas, Jianmin Cui, Jonathan R. Brestoff, Hong Chen
Nature Metabolism.2023; 5(5): 789. CrossRef - Changes in hypothalamic mu-opioid receptor expression following acute olanzapine treatment in female rats: Implications for feeding behavior
Maiken Krogsbaek, Nick Yao Larsen, Anne M. Landau, Connie Sanchez, Jens Randel Nyengaard
Journal of Chemical Neuroanatomy.2023; 132: 102324. CrossRef - Insulin Resistance and Glucose Metabolism during Infection
Borros Arneth
Endocrines.2023; 4(4): 685. CrossRef - The PACAP Paradox: Dynamic and Surprisingly Pleiotropic Actions in the Central Regulation of Energy Homeostasis
Nikki Le, Sarah Sayers, Veronica Mata-Pacheco, Edward J. Wagner
Frontiers in Endocrinology.2022;[Epub] CrossRef - The Link Between Energy-Related Sensations and Metabolism: Implications for Treating Fatigue
Marco Filippi, Rainer Krähenmann, Patrick Fissler
Frontiers in Psychology.2022;[Epub] CrossRef - Unaltered Tonic Inhibition in the Arcuate Nucleus of Diet-induced Obese Mice
Moonsun Sa, Jung Moo Lee, Mingu Gordon Park, Jiwoon Lim, Jong Min Joseph Kim, Wuhyun Koh, Bo-Eun Yoon, C. Justin Lee
Experimental Neurobiology.2022; 31(3): 147. CrossRef - Hypothalamus–Muscle Parallel Induction of Metabolic Pathways Following Physical Exercise
Almog Katz, Meital Gonen, Yael Shahar, Asael Roichman, Batia Lerrer, Haim Yosef Cohen
Frontiers in Neuroscience.2022;[Epub] CrossRef - Monocarboxylate transporters (MCTs) in skeletal muscle and hypothalamus of less or more physically active mice exposed to aerobic training
P.P.M. Scariot, F.B. Manchado-Gobatto, W.R. Beck, M. Papoti, P.R. Van Ginkel, C.A. Gobatto
Life Sciences.2022; 307: 120872. CrossRef - Obesity-Related Genes Expression in Testes and Sperm Parameters Respond to GLP-1 and Caloric Restriction
Ana S. Correia, Sara C. Pereira, Tiago Morais, Ana D. Martins, Mariana P. Monteiro, Marco G. Alves, Pedro F. Oliveira
Biomedicines.2022; 10(10): 2609. CrossRef - A pilot study of contrast-enhanced electrical impedance tomography for real-time imaging of cerebral perfusion
Yuyan Zhang, Jian’an Ye, Yang Jiao, Weirui Zhang, Tao Zhang, Xiang Tian, Xuetao Shi, Feng Fu, Liang Wang, Canhua Xu
Frontiers in Neuroscience.2022;[Epub] CrossRef - Repercussions of maternal exposure to high-fat diet on offspring feeding behavior and body composition: a systematic review
Wenicios Ferreira Chaves, Isabeli Lins Pinheiro, Jacqueline Maria da Silva, Raul Manhães-de-Castro, Raquel da Silva Aragão
Journal of Developmental Origins of Health and Disease.2021; 12(2): 220. CrossRef - Obesity-associated Pathways of Anthocyanins
Elif YILDIZ, Metin GULDAS, Pinar ELLERGEZEN, Asli Gul ACAR, Ozan GURBUZ
Food Science and Technology.2021; 41( suppl 1): 1. CrossRef - Prostaglandin in the ventromedial hypothalamus regulates peripheral glucose metabolism
Ming-Liang Lee, Hirokazu Matsunaga, Yuki Sugiura, Takahiro Hayasaka, Izumi Yamamoto, Taiga Ishimoto, Daigo Imoto, Makoto Suematsu, Norifumi Iijima, Kazuhiro Kimura, Sabrina Diano, Chitoku Toda
Nature Communications.2021;[Epub] CrossRef - Sleep and Cardiovascular Risk
Lyudmila Korostovtseva, Mikhail Bochkarev, Yurii Sviryaev
Sleep Medicine Clinics.2021; 16(3): 485. CrossRef - Evaluation and Management of Early Onset Genetic Obesity in Childhood
Sonali Malhotra, Ramya Sivasubramanian, Gitanjali Srivastava
Journal of Pediatric Genetics.2021; 10(03): 194. CrossRef - Gene expression atlas of energy balance brain regions
Maria Caterina De Rosa, Hannah J. Glover, George Stratigopoulos, Charles A. LeDuc, Qi Su, Yufeng Shen, Mark W. Sleeman, Wendy K. Chung, Rudolph L. Leibel, Judith Y. Altarejos, Claudia A. Doege
JCI Insight.2021;[Epub] CrossRef - New Peptides as Potential Players in the Crosstalk Between the Brain and Obesity, Metabolic and Cardiovascular Diseases
Magdalena Czerwińska, Katarzyna Czarzasta, Agnieszka Cudnoch-Jędrzejewska
Frontiers in Physiology.2021;[Epub] CrossRef - A putative role for lncRNAs in epigenetic regulation of memory
Ashleigh B. Irwin, Rudhab Bahabry, Farah D. Lubin
Neurochemistry International.2021; 150: 105184. CrossRef - Alteration of Relative Rates of Biodegradation and Regeneration of Cervical Spine Cartilage through the Restoration of Arterial Blood Flow Access to Rhomboid Fossa: A Hypothesis
Kirill V. Zhukov, Alexandre A. Vetcher, Bagrat A. Gasparuan, Alexander Y. Shishonin
Polymers.2021; 13(23): 4248. CrossRef - Placental NEGR1 DNA methylation is associated with BMI and neurodevelopment in preschool-age children
E Breton, V Gagné-Ouellet, K Thibeault, R Guérin, Rj Van Lieshout, P Perron, Mf Hivert, L Bouchard
Epigenetics.2020; 15(3): 323. CrossRef - The dorsomedial hypothalamus and nucleus of the solitary tract as key regulators in a rat model of chronic obesity
Chen Zhang, Pernille Barkholt, Jens Christian Nielsen, Ditte Dencker Thorbek, Kristoffer Rigbolt, Niels Vrang, David Paul Drucker Woldbye, Jacob Jelsing
Brain Research.2020; 1727: 146538. CrossRef - Hypothalamic NAD+-Sirtuin Axis: Function and Regulation
Eun Roh, Min-Seon Kim
Biomolecules.2020; 10(3): 396. CrossRef - The Rho/Rac Guanine Nucleotide Exchange Factor Vav1 Regulates Hif-1α and Glut-1 Expression and Glucose Uptake in the Brain
Jaewoo Hong, Yurim Kim, Sudhirkumar Yanpallewar, P. Charles Lin
International Journal of Molecular Sciences.2020; 21(4): 1341. CrossRef - Sirtuin (SIRT)-1: At the crossroads of puberty and metabolism
Carlos F. Aylwin, Alejandro Lomniczi
Current Opinion in Endocrine and Metabolic Research.2020; 14: 65. CrossRef - Metabolomics Reveals the Alteration of Metabolic Pathway by Alpha-Melanocyte-Stimulating Hormone in B16F10 Melanoma Cells
Seung-Ho Seo, Jae Kwon Jo, Eun-Ju Kim, Seong-Eun Park, Seo Yeon Shin, Kyung Mok Park, Hong-Seok Son
Molecules.2020; 25(15): 3384. CrossRef - Noninvasive real-time detection of cerebral blood perfusion in hemorrhagic shock rabbits based on whole-brain magnetic induction phase shift: an experimental study
Wencai Pan, Wei Zhuang, Yinbao Chong, Mingxin Qin, Yang Li, Jingjing Xiao, Qing Wang, Shihui Zhang, Shuanglin Zhao, Peng Zhao
Physiological Measurement.2020; 41(9): 095004. CrossRef - Neurochemical regulators of food behavior for pharmacological treatment of obesity: current status and future prospects
Gayane Sargis Vardanyan, Hasmik Samvel Harutyunyan, Michail Iosif Aghajanov, Ruben Sargis Vardanyan
Future Medicinal Chemistry.2020; 12(20): 1865. CrossRef - The Co-occurrence of Pediatric Obesity and ADHD: an Understanding of Shared Pathophysiology and Implications for Collaborative Management
Valerie M. O’Hara, Jennifer L. Curran, Nancy T. Browne
Current Obesity Reports.2020; 9(4): 451. CrossRef - Constitutive Androstane Receptor: A Peripheral and a Neurovascular Stress or Environmental Sensor
Fabiana Oliviero, Céline Lukowicz, Badreddine Boussadia, Isabel Forner-Piquer, Jean-Marc Pascussi, Nicola Marchi, Laila Mselli-Lakhal
Cells.2020; 9(11): 2426. CrossRef - Automated diffusion-based parcellation of the hypothalamus reveals subunit-specific associations with obesity
Melanie Spindler, Jale Özyurt, Christiane M. Thiel
Scientific Reports.2020;[Epub] CrossRef - SIRT1 in Astrocytes Regulates Glucose Metabolism and Reproductive Function
Irene Choi, Emily Rickert, Marina Fernandez, Nicholas J G Webster
Endocrinology.2019; 160(6): 1547. CrossRef - Hypothalamic mechanisms associated with corticotropin-releasing factor-induced anorexia in chicks
Jinxin Wang, Justin Matias, Elizabeth R. Gilbert, Tetsuya Tachibana, Mark A. Cline
Neuropeptides.2019; 74: 95. CrossRef - HMG-CoA synthase 2 drives brain metabolic reprogramming in cocaine exposure
Xue Shao, Yunxuan Tang, Hailei Long, Hui Gu, Jie Zhang, Pengchi Deng, Yinglan Zhao, Xiaobo Cen
Neuropharmacology.2019; 148: 377. CrossRef - The Effect of Feeding Behavior on Hypothalamus in Obese Type 2 Diabetic Rats with Glucagon-like Peptide-1 Receptor Agonist Intervention
Ke Lu, Xiaoyan Chen, Jianhua Yan, Xinchun Li, Chen Huang, Qi Wan, Xuelian Deng, Qiao Zou
Obesity Facts.2018; 11(3): 181. CrossRef - The Long-Term Impact of High Levels of Alpha-Melanocyte-Stimulating Hormone in Energy Balance Among Obese Adolescents
Ana Claudia Pelissari Kravchychyn, Raquel Munhoz da Silveira Campos, Flávia Campos Corgosinho, Deborah Cristina Landi Masquio, Sofia Emanuelle de Castro Ferreira Vicente, Yasmin Alaby Martins Ferreira, Patrícia Leão Silva, Aline de Piano Ganen, Lila Missa
Annals of Nutrition and Metabolism.2018; 72(4): 279. CrossRef - Psychopharmacological advances in eating disorders
Hubertus Himmerich, Janet Treasure
Expert Review of Clinical Pharmacology.2018; 11(1): 95. CrossRef - Food engineering into the XXI century
José Miguel Aguilera
AIChE Journal.2018; 64(1): 2. CrossRef - The Role of Glucagon-Like Peptide 1 (GLP1) in Type 3 Diabetes: GLP-1 Controls Insulin Resistance, Neuroinflammation and Neurogenesis in the Brain
Choon Bae, Juhyun Song
International Journal of Molecular Sciences.2017; 18(11): 2493. CrossRef - “I Am I and My Bacterial Circumstances”: Linking Gut Microbiome, Neurodevelopment, and Depression
Juan M. Lima-Ojeda, Rainer Rupprecht, Thomas C. Baghai
Frontiers in Psychiatry.2017;[Epub] CrossRef - Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity
Katharina Timper, Jens C. Brüning
Disease Models & Mechanisms.2017; 10(6): 679. CrossRef - Brain glucose metabolism: Role of Wnt signaling in the metabolic impairment in Alzheimer’s disease
Pedro Cisternas, Nibaldo C. Inestrosa
Neuroscience & Biobehavioral Reviews.2017; 80: 316. CrossRef - Astrocyte-Specific Deletion of Peroxisome-Proliferator Activated Receptor-γ Impairs Glucose Metabolism and Estrous Cycling in Female Mice
Marina O Fernandez, Katherine Hsueh, Hyun Tae Park, Consuelo Sauceda, Vicky Hwang, Deepak Kumar, Sun Kim, Emily Rickert, Sumana Mahata, Nicholas J G Webster
Journal of the Endocrine Society.2017; 1(11): 1332. CrossRef - Articles inEndocrinology and Metabolismin 2016
Won-Young Lee
Endocrinology and Metabolism.2017; 32(1): 62. CrossRef
- Hypothalamic AMP-Activated Protein Kinase as a Whole-Body Energy Sensor and Regulator

- Clinical Study
- Radiographic Characteristics of Adrenal Masses in Oncologic Patients
- Ji Hyun Lee, Eun Ky Kim, A Ram Hong, Eun Roh, Jae Hyun Bae, Jung Hee Kim, Chan Soo Shin, Seong Yeon Kim, Sang Wan Kim
- Endocrinol Metab. 2016;31(1):147-152. Published online March 16, 2016
- DOI: https://doi.org/10.3803/EnM.2016.31.1.147
- 4,418 View
- 36 Download
- 9 Web of Science
- 8 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background We aimed to assess the usefulness of pre-contrast Hounsfield unit (HU) and mass size on computed tomography to differentiate adrenal mass found incidentally in oncologic patients.
Methods From 2000 to 2012, 131 oncologic patients with adrenal incidentaloma were reviewed retrospectively. Receiver operating characteristic (ROC) curves were applied to determine the optimal cut-off value of the mean HU and size for detecting adrenal metastasis.
Results The median age was 18 years, and 80 patients were male. The initial mass size was 18 mm, and 71 (54.2%) of these were on the left side. A bilateral adrenal mass was found in 11 patients (8.4%). Biochemically functional masses were observed in 9.2% of patients. Thirty-six out of 119 patients with nonfunctional masses underwent adrenalectomy, which revealed metastasis in 13. The primary cancers were lung cancer (
n =4), renal cell carcinoma (n =2), lymphoma (n =2), hepatocellular carcinoma (n =2), breast cancer (n =1), and others (n =2). The area under the curve for the size and HU for clinically suspicious metastasis were 0.839 (95% confidence interval [CI], 0.761 to 0.900;P <0.001) and 0.959 (95% CI, 0.898 to 0.988;P <0.001), respectively. The cut-off value to distinguish between metastasis and benign masses were 22 mm for size and 20 for HU.Conclusion ROC curve results suggest that pre-contrast HU >20 can be used as a diagnostic reference to suggest metastasis in oncologic patients with adrenal masses.
-
Citations
Citations to this article as recorded by- Risk of malignancy in adrenal tumors in patients with a history of cancer
Radosław Samsel, Karolina Nowak, Lucyna Papierska, Edyta Karpeta, Katarzyna Roszkowska-Purska, Wacław Smiertka, Tomasz Ostrowski, Eryk Chrapowicki, Alan Grabowski, Dorota Leszczyńska, Andrzej Cichocki
Frontiers in Oncology.2023;[Epub] CrossRef - Adrenal Tumors Found During Staging and Surveillance for Colorectal Cancer: Benign Incidentalomas or Metastatic Disease?
Mio Yanagisawa, Dania G. Malik, Thomas W. Loehfelm, Ghaneh Fananapazir, Michael T. Corwin, Michael J. Campbell
World Journal of Surgery.2020; 44(7): 2282. CrossRef - Predictive factors for adrenal metastasis in extra‐adrenal malignancy patients with solitary adrenal mass
Kyeong‐Hyeon Byeon, Yun‐Sok Ha, Seock‐Hwan Choi, Bum Soo Kim, Hyun Tae Kim, Eun Sang Yoo, Tae Gyun Kwon, Jun Nyung Lee, Tae‐Hwan Kim
Journal of Surgical Oncology.2018; 118(8): 1271. CrossRef - Combining Washout and Noncontrast Data From Adrenal Protocol CT
Chaan S. Ng, Emre Altinmakas, Wei Wei, Payel Ghosh, Xiao Li, Elizabeth G. Grubbs, Nancy A. Perrier, Victor G. Prieto, Jeffrey E. Lee, Brian P. Hobbs
Academic Radiology.2018; 25(7): 861. CrossRef - Evaluation of quantitative parameters for distinguishing pheochromocytoma from other adrenal tumors
Youichi Ohno, Masakatsu Sone, Daisuke Taura, Toshinari Yamasaki, Katsutoshi Kojima, Kyoko Honda-Kohmo, Yorihide Fukuda, Koji Matsuo, Toshihito Fujii, Akihiro Yasoda, Osamu Ogawa, Nobuya Inagaki
Hypertension Research.2018; 41(3): 165. CrossRef - Articles inEndocrinology and Metabolismin 2016
Won-Young Lee
Endocrinology and Metabolism.2017; 32(1): 62. CrossRef - The Diverse Clinical Presentations of Adrenal Lymphoma
Awais Masood, Anna Tumyan, Daniel R. Nussenzveig, Dara N. Wakefield, Diana Barb, Hans K. Ghayee, Naim M. Maalouf
AACE Clinical Case Reports.2017; 3(4): 307. CrossRef - Optimal follow-up strategies for adrenal incidentalomas: reappraisal of the 2016 ESE-ENSAT guidelines in real clinical practice
A Ram Hong, Jung Hee Kim, Kyeong Seon Park, Kyong Young Kim, Ji Hyun Lee, Sung Hye Kong, Seo Young Lee, Chan Soo Shin, Sang Wan Kim, Seong Yeon Kim
European Journal of Endocrinology.2017; 177(6): 475. CrossRef
- Risk of malignancy in adrenal tumors in patients with a history of cancer

- Thyroid
- Two Cases of Methimazole-Induced Insulin Autoimmune Syndrome in Graves' Disease
- Eun Roh, Ye An Kim, Eu Jeong Ku, Jae Hyun Bae, Hye Mi Kim, Young Min Cho, Young Joo Park, Kyong Soo Park, Seong Yeon Kim, Soo Heon Kwak
- Endocrinol Metab. 2013;28(1):55-60. Published online March 25, 2013
- DOI: https://doi.org/10.3803/EnM.2013.28.1.55
- 6,380 View
- 71 Download
- 15 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader We report here the cases of two females with Graves' disease who developed insulin autoimmune syndrome after treatment with methimazole. The patients exhibited a sudden altered mental state after treatment with methimazole for approximately 4 weeks. Patients had hypoglycemia with serum glucose below 70 mg/dL, and laboratory findings showed both high levels of serum insulin and high titers of insulin autoantibodies. The two women had never been exposed to insulin or oral antidiabetic agents, and there was no evidence of insulinoma in imaging studies. After glucose loading, serum glucose, and total insulin levels increased abnormally. One of the patient was found to have HLA-DRB1*0406, which is known to be strongly associated with methimazole-induced insulin autoimmune syndrome. After discontinuation of methimazole, hypoglycemic events disappeared within 1 month. Insulin autoantibody titer and insulin levels decreased within 5 months and there was no further development of hypoglycemic events. We present these cases with a review of the relevant literature.
-
Citations
Citations to this article as recorded by- Insulin Autoimmune Syndrome: A Systematic Review
MingXu Lin, YuHua Chen, Jie Ning, Tatsuya Kin
International Journal of Endocrinology.2023; 2023: 1. CrossRef - Safety of Antithyroid Drugs in Avoiding Hyperglycemia or Hypoglycemia in Patients With Graves’ Disease and Type 2 Diabetes Mellitus: A Literature Review
Yu-Shan Hsieh
Cureus.2023;[Epub] CrossRef - Case report: hypoglycemia secondary to methimazole-induced insulin autoimmune syndrome in young Taiwanese woman with Graves’ disease
Hsuan-Yu Wu, I-Hua Chen, Mei-Yueh Lee
Medicine.2022; 101(25): e29337. CrossRef - Analysis of the clinical characteristics of insulin autoimmune syndrome induced by methimazole
Linli Sun, Weijin Fang, Dan Yi, Wei Sun, Chunjiang Wang
Journal of Clinical Pharmacy and Therapeutics.2021; 46(2): 470. CrossRef - Preoperative plasmapheresis experience in Graves’ disease patients with anti-thyroid drug-induced hepatotoxicity
Tugce Apaydın, Onur Elbasan, Dilek Gogas Yavuz
Transfusion and Apheresis Science.2020; 59(5): 102826. CrossRef - Glycemic variation in uncontrolled Graves’ disease patients with normal glucose metabolism: Assessment by continuous glucose monitoring
Gu Gao, Feng-fei Li, Yun Hu, Reng-na Yan, Bing-li Liu, Xiao-mei Liu, Xiao-fei Su, Jian-hua Ma, Gang Hu
Endocrine.2019; 64(2): 265. CrossRef - Insulin autoimmune syndrome induced by exogenous insulin injection: a four-case series
Yimin Shen, Xiaoxiao Song, Yuezhong Ren
BMC Endocrine Disorders.2019;[Epub] CrossRef - Assessment and Management of Anti-Insulin Autoantibodies in Varying Presentations of Insulin Autoimmune Syndrome
David Church, Luís Cardoso, Richard G Kay, Claire L Williams, Bernard Freudenthal, Catriona Clarke, Julie Harris, Myuri Moorthy, Efthmia Karra, Fiona M Gribble, Frank Reimann, Keith Burling, Alistair J K Williams, Alia Munir, T Hugh Jones, Dagmar Führer,
The Journal of Clinical Endocrinology & Metabolism.2018; 103(10): 3845. CrossRef - MANAGEMENT OF ENDOCRINE DISEASE: Pathogenesis and management of hypoglycemia
Nana Esi Kittah, Adrian Vella
European Journal of Endocrinology.2017; 177(1): R37. CrossRef - Insulin autoimmune syndrome during the administration of clopidogrel
Eijiro Yamada, Shuichi Okada, Tsugumichi Saito, Aya Osaki, Atushi Ozawa, Masanobu Yamada
Journal of Diabetes.2016; 8(4): 588. CrossRef - Hyperinsulinemic hypoglycemia associated with insulin antibodies caused by exogenous insulin analog
Chih-Ting Su, Yi-Chun Lin
Endocrinology, Diabetes & Metabolism Case Reports.2016;[Epub] CrossRef - Anti-tuberculosis Treatment-Induced Insulin Autoimmune Syndrome
Jung Suk Han, Han Ju Moon, Jin Seo Kim, Hong Il Kim, Cheol Hyeon Kim, Min Joo Kim
The Ewha Medical Journal.2016; 39(4): 122. CrossRef - 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis
Douglas S. Ross, Henry B. Burch, David S. Cooper, M. Carol Greenlee, Peter Laurberg, Ana Luiza Maia, Scott A. Rivkees, Mary Samuels, Julie Ann Sosa, Marius N. Stan, Martin A. Walter
Thyroid.2016; 26(10): 1343. CrossRef - Insulin Autoimmune Syndrome in a Patient with Hashimoto's Thyroiditis
In Wook Song, Eugene Han, Nan Hee Cho, Ho Chan Cho
Journal of Korean Thyroid Association.2014; 7(2): 180. CrossRef - Brief Review of Articles in 'Endocrinology and Metabolism' in 2013
Won-Young Lee
Endocrinology and Metabolism.2014; 29(3): 251. CrossRef
- Insulin Autoimmune Syndrome: A Systematic Review

- Characterization of Incidentally Detected Adrenal Pheochromocytoma.
- Ye An Kim, Yul Hwangbo, Min Joo Kim, Hyung Jin Choi, Je Hyun Seo, Yenna Lee, Soo Heun Kwak, Eu Jeong Ku, Tae Jung Oh, Eun Roh, Jae Hyun Bae, Jung Hee Kim, Kyoung Soo Park, Seong Yeon Kim
- Endocrinol Metab. 2012;27(2):132-137. Published online June 20, 2012
- DOI: https://doi.org/10.3803/EnM.2012.27.2.132
- 2,286 View
- 28 Download
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
In approach to an adrenal incidentaloma, early exclusion of pheochromocytoma is clinically important, due to the risk of catecholamine crisis. The aims of this study are to investigate the characteristics of incidentally detected pheochromocytomas, compared with that of the other adrenal incidentalomas, and to compare these characteristics with those of symptomatic pheochromocytomas. METHODS: In this retrospective study, we reviewed the medical records of 198 patients with adrenal incidentaloma from 2001 to 2010. We analyzed the clinical, laboratory and radiological data of pheochromocytomas, in comparison with those of the other adrenal incidentalomas. We also compared the characteristics of these incidentally detected pheochromocytomas with the medical records of 28 pathologically proven pheochromocytomas, diagnosed based on typical symptoms. RESULTS: Among the 198 patients with adrenal incidentaloma, nineteen patients were diagnosed with pheochromocytoma. Pheochromocytomas showed larger size and higher Hounsfield unit at precontrast computed tomography (CT) than did non-pheochromocytomas. All pheochromocytomas were larger than 2.0 cm, and the Hounsfield units were 19 or higher in precontrast CT. When both criteria of size > 2.0 cm and Hounsfield unit > 19 were met, the sensitivity and specificity for the diagnosis of pheochromocytoma were 100% and 79.3%, respectively. Compared with patients with pheochromocytoma, diagnosed based on typical symptoms, patients with incidentally detected pheochromocytoma were older, presented less often with hypertension, and showed lower levels of 24-hour urine metanephrine. CONCLUSION: Adrenal incidentaloma with < 2.0 cm in size or < or = 19 Hounsfield units in precontrast CT imaging was less likely to be a pheochromocytoma. Patients with incidentally discovered pheochromocytoma showed lower catecholamine metabolites, compared with those patients with symptomatic pheochromocytoma. -
Citations
Citations to this article as recorded by- Guidelines for the Management of Adrenal Incidentaloma: the Korean Endocrine Society, Committee of Clinical Practice Guidelines
Jung-Min Lee, Mee Kyoung Kim, Seung-Hyun Ko, Jung-Min Koh, Bo-Yeon Kim, Sang-Wan Kim, Soo-Kyung Kim, Hae-Jin Kim, Ohk-Hyun Ryu, Juri Park, Jung-Soo Lim, Seong Yeon Kim, Young Kee Shong, Soon Jib Yoo
The Korean Journal of Medicine.2017; 92(1): 4. CrossRef - Clinical Guidelines for the Management of Adrenal Incidentaloma
Jung-Min Lee, Mee Kyoung Kim, Seung-Hyun Ko, Jung-Min Koh, Bo-Yeon Kim, Sang Wan Kim, Soo-Kyung Kim, Hae Jin Kim, Ohk-Hyun Ryu, Juri Park, Jung Soo Lim, Seong Yeon Kim, Young Kee Shong, Soon Jib Yoo
Endocrinology and Metabolism.2017; 32(2): 200. CrossRef - Characterization of Incidentally Detected Adrenal Pheochromocytoma
Soon Jib Yoo, Woohyeon Kim
Endocrinology and Metabolism.2012; 27(2): 116. CrossRef
- Guidelines for the Management of Adrenal Incidentaloma: the Korean Endocrine Society, Committee of Clinical Practice Guidelines


 KES
KES

 First
First Prev
Prev



